INTRODUCTION
Blood transfusion may be associated with adverse events, which range from incidents that cause no harm to others that result in life-threatening or even fatal complications for the patient. Various frameworks have been developed (e.g., International Haemovigilance Network [IHN] / International Society of Blood Transfusion [ISBT], Serious Hazards of Transfusion [SHOT], Transfusion and Transplantation Reactions in Patients [TRIP] data or National Healthcare Safety Network [NHSN]), which serve as excellent resources for background data and structured definitions pertaining to transfusion-associated adverse events [1–4]. All transfusion-related adverse events require timely assessment and intervention, as guided by the type of event. Where possible, measures should be taken to prevent recurrence in the future. Reporting of adverse events is important, both locally (i.e., at a hospital level), as well nationally (i.e., to haemovigilance programmes), where such programmes exist. Despite existing safeguards, human error is responsible for a large proportion of serious and even fatal transfusion-associated adverse events. Online training programmes on good transfusion practice are available and can help to mitigate risk to transfusion recipients [5]. A World Health Organization (WHO) Aide Memoire on National Haemovigilance systems can be found on the WHO website at: https://apps.who.int/iris/handle/10665/340564.
SIGNS AND SYMPTOMS OF TRANSFUSION REACTION
Transfusion reactions often have overlapping signs and symptoms; therefore, it is imperative that medical, nursing and laboratory personnel be aware of the signs and symptoms of the different reactions as well as potential diagnostic overlap to ensure timely and appropriate management (Table 1).
Abbreviations: AHTR, acute haemolytic transfusion reaction; DIC, disseminated intravascular coagulation; FNHTR, febrile non-haemolytic transfusion reaction; TACO, transfusion-associated circulatory overload; TAD, transfusion-associated dyspnoea; TRALI, transfusion-related acute lung injury.
Table 1. Signs and symptoms of acute transfusion reactions with differential diagnosis. This list can be used as a guide and may not be inclusive. Always consider non-transfusion-related causes.
|
Sign /symptoms |
Type of reaction |
Comment |
|
Fever (temperature of ≥38oC AND rise of ≥1oC from baseline) |
FNHTR, AHTR, TRALI, bacterial contamination, can be unrelated to the blood transfusion |
Can coexist with other signs such as chills, rigors, myalgia, nausea or vomiting, dyspnoea, hypotension and tachycardia |
|
Urticaria, hives, pruritus |
Allergic transfusion reaction, anaphylaxis |
Can be mild and localized or more severe with generalized urticaria |
|
Dyspnoea or hypoxia
|
TRALI, TACO, TAD, severe allergic transfusion reaction, anaphylaxis, sepsis |
Severe dyspnoea without shock may occur in TRALI or TACO. TAD is a diagnosis of exclusion, therefore, patients should be assessed for other causes of dyspnoea before making this diagnosis |
|
Stridor, bronchospasm |
Allergic/anaphylaxis |
|
|
Pulmonary oedema |
TACO, TRALI, sepsis |
|
|
Angioedema |
Allergic transfusion reaction |
May be preceded by tingling around the face and lips |
|
Hypotension (fall in systolic and/or diastolic blood pressure by >30 mmHg and systolic blood pressure of ≤80 mmHg) |
AHTR, severe allergic transfusion reaction, anaphylaxis, bacterial contamination, TRALI, hypotensive reaction (bradykinin-mediated hypotension) |
Patients on ACE inhibitors are at risk. Risk is higher with bedside leukoreduction. Isolated hypotensive reactions are a diagnosis of exclusion, and occur within an hour of transfusion, in the absence of allergic or anaphylactic symptoms. These reactions usually require no/minor intervention. |
|
Pain |
FNHTR (generalized aches), AHTR (pain at the infusion site, abdomen, chest, loins) |
|
|
Bleeding diathesis with acute onset |
DIC can be associated with AHTR, bacterial contamination |
|
CLASSIFICATION OF TRANSFUSION REACTIONS
Transfusion reactions can be classified clinically by:
1. Timing and speed of onset (i.e., acute or delayed)
- Acute transfusion reactions occur within minutes and up to 24 h from the commencement of the transfusion.
- Delayed adverse events manifest themselves more than 24 h from the index transfusion, even if the pathophysiology commences earlier. The consequences of some adverse events may only become evident after many years (e.g., iron overload in chronically transfused patients and some transfusion-transmitted infectious diseases [TTIDs]).
2. Severity (i.e., non-severe, severe, life-threatening and death) [1]
- Non-severe transfusion reactions are self-limiting and/or resolve with non-invasive intervention, for example, mild fever or urticaria.
- Severe events require hospitalization or incur prolongation of hospitalization due to the reaction.
- Life-threatening events require major medical intervention to prevent death, or result in death.
3. Pathological process (i.e., immune vs. non-immune)
- Immunological reactions span major and minor acute haemolytic transfusion reactions (AHTRs), delayed haemolytic transfusion reactions (DHTRs), alloimmunization (i.e., development of antibodies against red cell, white cell or platelet antigens), allergic transfusion reaction (including anaphylaxis), febrile non-haemolytic transfusion reactions (FNHTRs) and transfusion-related acute lung injury (TRALI).
- Non-immune complications of blood transfusion include:
- Infectious sequelae: acute (e.g., bacterial contamination leading to sepsis) or delayed (e.g., cirrhosis following transfusion-transmitted hepatitis viruses);
- Mechanical complications: haemolysis from improper blood component storage or infusion thorugh malfunctioning blood warmers;
- Other: circulatory overload.
ASSESSMENT, INVESTIGATION AND MANAGEMENT
Patients who undergo blood transfusion must be monitored both during and after the transfusion. Early recognition of an adverse event enables timely treatment and investigation. Unconscious, anaesthetized or very young patients (such as neonates and infants) require a high level of vigilance as they cannot describe their symptoms. There should be careful attention to changes in vital signs, cardiorespiratory status, or evidence of haemolysis and/or bleeding. Some features may be obscured by the patient’s underlying condition or treatment. For example, fever, a common sign of an acute transfusion reaction, may be blunted in the presence of antipyretics or antibiotics. Fever can also be caused by the patient’s underlying disease (e.g., neutropenic fever in a patient receiving chemotherapy). Other, unrelated events (e.g., pulmonary embolus, drug reaction, etc.) may be the cause of new signs and symptoms, so it is important to consider a broad differential diagnosis when investigating suspected transfusion-associated adverse events. However, any sign or symptom that is temporally related to transfusion (i.e., occurs during or around the time of or following transfusion) warrants investigation.
Clinical assessment and management
Any patient with a new symptom or sign that is temporally related to transfusion requires immediate evaluation and timely intervention, as guided by the clinical assessment.
Figure 1 summarizes the general approach to the management of an acute transfusion reaction. In addition to the steps outlined in Figure 1, further interventions that should be undertaken at each step include:
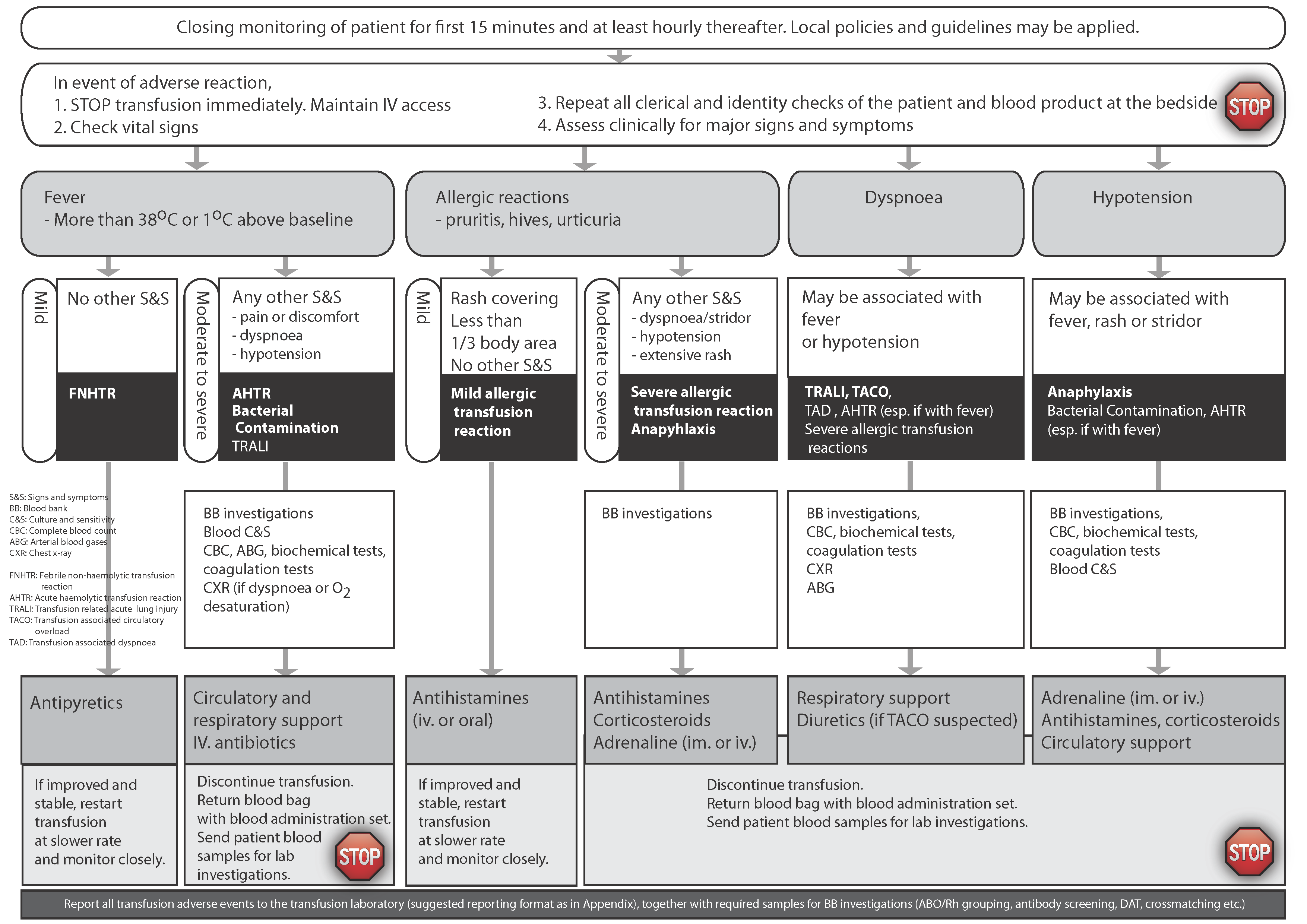
Figure 2. Example of a Transfusion Reaction Notification form.
Reporting
Hospitals should have a mechanism to document the patient’s clinical presentation, management and investigation of the event. This also enables reporting to local or national haemovigilance programmes.
All incidents should be reviewed by both the transfusing physician and the blood bank physician. In addition there should be oversight of adverse events by the local transfusion committee. Collectively, this enables determination of the cause and contributing factors for the event as well as recommendations regarding corrective and preventive actions.
The findings of the investigation should be discussed with the patient (sometimes called ‘open disclosure’). Patients should also be notified if the investigation yields information that could affect their future transfusions and/or pregnancies (e.g., development of a new red blood cell [RBC] antibody).
ACUTE TRANSFUSION REACTIONS
Key point 3: Correct identification of donor and recipient is critical in transfusion practice.
Acute haemolytic transfusion reaction (AHTR)
Pathophysiology
- AHTR most commonly results from transfusion of red cells which are serologically incompatible with the patient.
- Transfusion of incompatible red cells can lead to complement activation and intravascular haemolysis.
- Major AHTR is ascribed to pre-existing antibodies in the patient’s plasma binding to donor RBCs. This is most commonly attributed to ABO-mismatched red cell transfusion (e.g., group A red cell administered to a group O recipient) caused by human error.
- Anti-A and -B antibodies are predominantly IgM and complement-binding, and therefore capable of causing massive intravascular haemolysis, leading to cytokine and membrane phospholipid release, haemoglobinaemia and nitrous oxide scavenging. This may lead to renal failure and disseminated intravascular coagulation (DIC).
- Antibodies against antigens of other blood group systems, such as anti-Jka, may also cause intravascular haemolysis.
- Immunoglobulin (Ig)G antibodies can also be implicated in causing an AHTR, although they tend not to fix complement so the presentation is usually different.
- Rarely, ABO-associated AHTR is caused by incompatible plasma present in apheresis platelet concentrates or whole blood if ‘minor’ incompatible units are used in a large-volume transfusion (e.g., group O platelets transfused to a group A recipient).
- The most common cause of ABO-incompatible transfusion is human error. Examples include:
- Clerical errors on the blood request form;
- Mis-identification of the intended patient and/or mislabelling of pre-transfusion blood samples (wrong blood in tube errors) at the time of pre-transfusion sample collection. These errors are more likely to occur when pre-transfusion samples are not labelled at the patient’s bedside immediately after sample collection;
- Incorrect blood grouping, compatibility testing or labelling of the components in the laboratory;
- Inadequate bedside checks of the units against the patient’s identity wrist-band before starting the transfusion.
Even a small volume (10–50 mL) of incompatible blood can cause a severe or even fatal reaction, and larger volumes amplify the risks.
Signs and symptoms
- Clinical presentation is variable. It is possible for patients to develop intravascular haemolysis without signs or symptoms of a reaction. Therefore, the absence of classic signs and symptoms does not exclude the diagnosis of AHTR. Common symptoms/signs of an AHTR include:
- Fever, back, flank or loin pain, discomfort and hypotension;
- Chills, rigor, myalgia, nausea and/or vomiting;
- Pain at the infusion site, feelings of ‘impending doom’, anxiety and facial flushing;
- Dark (‘Coca-Cola’) urine due to haemoglobinuria.
- Laboratory evidence of intravascular haemolysis may include:
- An unexplained decline in haemoglobin (Hb) level;
- Positive direct antiglobulin test (DAT; Coombs’ test);
- Elevation in serum lactate dehydrogenase (LDH) and bilirubin (total and indirect);
- Decrease in haptoglobin (may be undetectable);
- Haemoglobinuria;
- DIC.
- In the absence of early recognition and intervention, AHTR can progress rapidly to acute renal failure, DIC and even death. Therefore, AHTR should be considered as a medical emergency.
- The differential diagnosis includes other severe transfusion reactions (e.g., bacterial contamination) as well as underlying disease.
Treatment and investigations
In cases of AHTR:
- Call for additional help—patients can deteriorate quickly, and may need to be transferred to an intensive care setting;
- Ensure a new intravenous line is established and start 0.9% normal saline;
- Maintain fluid balance;
- Ensure that renal function and sufficient diuresis is maintained. The use of diuretics could be considered to limit renal damage;
- In case of renal failure, consider urine alkalinization and/or renal dialysis;
- In acute serious cases, consider exchange RBC transfusion;
- Treat DIC as guided by coagulation test results.
- Laboratory investigations: Immediate post-transfusion blood samples (one clotted and one EDTA anticoagulated) must be collected from the arm opposite to the infusion site and sent to the laboratory. The blood unit and infusion set containing red cells from the transfused donor blood must also be returned to the laboratory. The first urine sample immediately following the reaction must also be collected and submitted to the laboratory. Evidence of haemolysis may be present on gross (i.e., macroscopic) inspection of the tubes, as reflected by pink discolouration of the plasma following centrifugation.
- The following tests must be performed if possible using the original pre- and new post-transfusion samples:
- Repeat ABO and Rh(D) group of the patient;
- Repeat ABO and Rh(D) group on the blood unit(s);
- Repeat antibody screen and serological crossmatches;
- DAT;
- Complete blood count;
- Coagulation tests, for example, PT, activated partial tromboplastin time, fibrinogen, D-dimer;
- Tests for haemolysis, for example, LDH, bilirubin (total and indirect), haptoglobin, plasma haemoglobin;
- Urea, creatinine and electrolytes;
- Gram stain, blood culture and sensitivity on the blood unit (to exclude bacterial contamination);
- In the case of dyspnoea, a chest X-ray must be requested.
- As soon as a reaction is suspected, the hospital blood bank should be informed, immediately by telephone as well as via routine reporting procedures. This is crucial to quarantine and recall any co-components that could have been issued to incorrect patients as a result of a mix-up of samples or units.
Prevention
- Ensure proper patient identification (check wrist-band and ask the patient to state their name and date of birth) prior to collecting pre-transfusion samples.
- Bedside sample labelling (before leaving the patient) is mandatory to avoid sample mislabelling.
- Label all blood samples correctly, and request forms with unique patient identifiers.
- The blood bank should adhere to strict rules regarding acceptability of blood samples for pre-transfusion evaluation.
- Always check the blood bag against the identity of the patient at the bedside before initiation of the transfusion.
- Pre-transfusion testing must include an antibody screen. If the antibody screen is positive, antibody identification should be performed. If a clinically significant antibody is identified in the patient, units selected for the transfusion should be antigen-negative for the implicated antibody, and must be serologically crossmatch-compatible with the patient’s plasma using an indirect antiglobulin method.
Bacterial contamination (septic tranfusion reaction)
Key point 4: Donor skin disinfection and aseptic technique at time of blood collection reduce the risk of bacterial contamination of blood components.
Pathophysiology
Blood components may become contaminated in any of the following ways:
- Non-adherence to aseptic techniques during donor phlebotomy, leading to introduction of donor bacteria (typically skin and mucosal flora) into the donated unit. Note that even careful adherence to proper techniques does not eliminate all risk—skin disinfection does remove all bacteria from the skin or skin appendages through which the phlebotomy needle must pass to reach the donor’s vein;
- Asymptomatic bacteraemia in the donor at the time of blood donation (e.g., Yersinia);
- Improper handling (lack of closed system) during blood processing;
- Incorrect storage conditions;
- Defects or damage to the plastic blood pack;
- Thawing fresh frozen plasma or cryoprecipitate in a water-bath which has not been properly decontaminated and/or without protecting the infusion ports on the bags, which may allow bacteria to enter through tiny cracks in the plastic bag.
Epidemiology
- Worldwide, this is a major transfusion risk and can occur anywhere.
- Platelets are at the highest risk of contamination given their routine storage at room temperature in gas-permeable bags:
- Most common are Gram-positive skin or mucosal flora, such as Streptococcus spp., coagulase-negative Staphylococcus spp. (e.g., S. epidermidis) and S. aureus;
- Environmental pathogens (e.g., Bacillus spp.).
- RBCs:
- Most risk from Gram-negative organisms that grow at 2–6°C such as Y. enterocolitica, E. coli, Klebsiella spp., Pseudomonas spp. and Serratia spp.
Signs and symptoms
- Clinical presentation is highly variable and may be masked by concurrent antibiotic use or other medications (e.g., steroids, antipyretics).
- Signs of a septic transfusion reaction may include fever, chills, rigors, hypotension, shock, nausea or vomiting.
- Bacterial contamination should be considered if fever is persistent or refractory to symptomatic measures (e.g., antipyretics).
- Although symptoms and signs typically develop soon after starting the infusion if left untreated, severe complications (e.g., septic shock, DIC) can occur; septic transfusion reactions can be fatal.
Treatment
- Bacterial contamination/septic transfusion reaction is a medical emergency.
- Stop the transfusion.
- Call for additional help if required.
- The implicated unit should be inspected for any discolouration, abnormal particles, clumps or signs of leakage. However, most bacterially contaminated units appear unremarkable, and absence of these features does not exclude the possibility that the unit is contaminated. If bacterial contamination is suspected, the hospital blood bank must be notified immediately so its staff may recall and quarantine any other components from the implicated donation.
- The implicated unit should be sent for urgent Gram stain and bacterial culture.
- Blood cultures should be drawn from the patient.
- Commence broad-spectrum antibiotics immediately after collecting patient blood cultures.
- Manage complications (e.g., DIC, shock).
Prevention
- Careful donor screening and selection of donors.
- Standardized disinfection of donor skin prior to blood donation with approved disinfectants.
- Use of a diversion pouch during blood donation whenever possible.
- Adherence to aseptic techniques in unit handling and processing.
- Adherence to sterile and aseptic transfusion procedures.
- Adherence to appropriate storage conditions for blood products (e.g., do not exceed shelf-life, maintain in temperature-controlled environment).
Febrile Non-Haemolytic Transfusion Reaction
Key point 5: FNHTR is a common adverse effect of blood transfusion that can be reduced by leucoreduction. Always consider whether fever could be a manifestation of a more serious transfusion-related adverse event before diagnosing an FNHTR.
FNHTR is one of the commonest forms of transfusion reaction. Fever, chills and rigors can be unpleasant and unsettling for patients. In addition, these reactions can cause interruptions and delays to patient care while being investigated. The challenge for clinical and laboratory staff is to exclude other more serious causes for the patient’s symptoms and signs in a timely manner, and to determine whether the transfusion can continue.
Pathophysiology
- FNHTRs are caused by inflammatory cytokines in the donor product or antibodies (e.g., human leucocyte antigen [HLA]) present in the patient that interact with donor leucocytes in the blood product. The incidence of FNHTR may be reduced by leucoreduction, which is more effective when undertaken prior to storage rather than at the bedside or at the time of issue from the blood bank. However, leucoreduction does not eliminate the risk of FNHTR.
- The diagnosis of FNHTR is made by exclusion of other causes.
Signs and symptoms
The most prominent symptoms of FNHTR include:
- Fever;
- Chills, rigors;
- Anxiety, tachycardia.
Differential diagnosis includes:
- AHTR;
- Bacterial contamination/septic transfusion reaction;
- Underlying disease (i.e. unrelated to transfusion).
Laboratory investigation of FNHTR should be guided by the need to exclude the above.
Treatment
- Treatment is symptomatic, for example, antipyretics.
- Transfusion of the remainder of the implicated components is usually not recommended but can be considered after careful evaluation and with close monitoring.
Prevention
Leucoreduction of cellular blood products (pre-storage, or bedside if the former is not available). Pre-medication with anti-pyretics before the transfusion might also help to prevent minor temperature fluctuations, especially if the patient has a history of recurrent events.
Allergic transfusion reaction
Pathophysiology
Signs and symptoms
Allergic reactions have a wide spectrum, from mild (localized urticaria) to severe. The latter may be life-threatening. Mild allergic transfusion reactions are very common, particularly with blood products that contain a high volume of plasma (e.g., plasma, platelets). Severe and anaphylactic reactions are much less common but can be life-threatening or even fatal in the absence of timely intervention.
- Mild allergic reactions typically manifest as localized skin rashes/urticarial (itching) in the absence of systemic symptoms.
- Severe/life-threatening/anaphylactic allergic transfusion reactions: In addition to muco-cutaneous signs (i.e., rash/urticaria), severe/life-threatening/anaphylactic reactions manifest respiratory (e.g., throat tightness, bronchospasms, wheezing, etc.) and/or cardiovascular signs (e.g., hypotension, cardiorespiratory collapse).
Treatment
- Mild allergic reactions: Initiate general measures for management of transfusion reactions. Anti-histamines may be given; if assessed to be a mild allergic reaction, the transfusion may possibly be restarted at a slower rate of infusion and under close observation of the patient.
- Severe and life-threatening allergic reactions (see Anaphylactic transfusion reaction below): Ensure that airway remains patent; any of the following may be considered depending on presentation: epinephrine (adrenaline), oxygen, bronchodilators, antihistamines.
- Call for additional help if required—patients may deteriorate quickly.
Prevention
- In the case of patients with recurrent allergic reactions, pre-medication with anti-histamines prior to transfusion may be considered.
- In cases of severe and/or recurrent allergic reactions with worsening symptoms, washed cellular blood products may be considered. Washing involves reducing the volume of donor plasma in the product to decrease the donor proteins in the blood product. The washing process is time-consuming and leads to variable loss of cells, particularly with platelet components.
Severe allergic transfusion reaction /anaphylactic reaction
Pathophysiology
Anaphylaxis is a severe, life-threatening hypersensitivity reaction. Deficiencies of IgA or haptoglobin are two (of many) possible causes of recurrent anaphylactic transfusion reactions.
Signs and symptoms
- Symptoms may develop very soon after commencement of the transfusion:
- Severe hypotension or cardiorespiratory collapse;
- Bronchospasm and angioedema, which may progress rapidly to airway and cardiorespiratory compromise;
- May or may not be preceded by urticaria.
- Anaphylactic transfusion reaction is a clinical diagnosis and does not rely on laboratory investigation.
- The differential diagnosis includes:
- Bacterial contamination/septic transfusion reaction;
- AHTR;
- Underlying disease and/or other causes of anaphylaxis (e.g., medications).
Note: Fever is not typical of allergic and/or anaphylactic transfusion reactions. This may help to distinguish these reactions from other differential diagnoses.
Treatment
This is a medical emergency that requires immediate intervention:
- Stop the transfusion;
- Call for additional help;
- Maintain airway and provide supplemental oxygen;
- Bronchodilators;
- Epinephrine (intramuscular injection or intravenous);
- Cardiorespiratory support;
- Anti-histamines and corticosteroids are probably of little use in the acute setting.
Prevention
- Pre-medication with steroids and anti-histamines and washing of cellular blood products.
- If subsequent transfusions are required, these should only be carried out where patients can be observed directly and where staff trained in managing anaphylaxis are available.
- A deficiency of IgA (with anti-IgA antibodies) or haptoglobin accounts for a small proportion of anaphylactic transfusion reactions.
- Some blood establishments have blood products available from IgA-deficient platelet or plasma donors.
- The removal of plasma through washing of cellular blood products can be considered to prevent recurrence of anaphylactic transfusion reactions.
Transfusion-Related Acute Lung Injury (TRALI)
Key point 6: Respiratory complications of transfusion can be serious and occur suddenly. They may be difficult to distinguish from each other initially and require careful clinical assessment.
- TRALI is defined as non-cardiogenic pulmonary oedema occurring within 6 h of commencing transfusion.
- TRALI has been most commonly associated with plasma units but can occur with any plasma-containing blood product.
- TRALI is a major cause of transfusion-associated fatality.
Pathophysiology
- At the time of writing, the definition of TRALI for international haemovigilance purposes is currently under review.
- The pathophysiology of TRALI remains incompletely understood. A ‘two-hit’ mechanism is possibly involved, where one hit is related to the blood product containing donor leucocyte antibodies and biological response modifiers, while the second hit is the patient’s underlying condition that results in pulmonary endothelial activation and neutrophil sequestration, making the patient more susceptible to TRALI.
- In this model, TRALI likely results from the interaction of specific leucocyte antibodies present in the transfused blood products reacting with the recipient’s pulmonary endothelium. Class I and II HLA antibodies as well as less commonly human neutrophil antibodies have also been implicated.
- The immunological response to leucocyte antibodies results in capillary endothelial damage, plasma leakage and a clinical picture of acute respiratory distress syndrome.
Signs and symptoms
- Symptoms include dyspnoea and hypoxaemia. Sometimes there is an associated fever and/or hypotension.
- New pulmonary infiltrates on chest X-ray (CXR).
- TRALI is a clinical diagnosis, supported by CXR, and exclusion of circulatory overload.
- The differential diagnosis includes:
- Transfusion-associated circulatory overload (TACO): If available, the measurement of B-natriuretic peptide (BNP) and NT-pro-BNP may be useful in differentiating TACO from TRALI.
- Severe allergic or anaphylactic reaction.
Treatment
- Stop the transfusion and ensure that the patient’s airway is maintained.
- Treatment of TRALI is symptomatic with pulmonary support: maintenance of the patient’s airway and administration of oxygen.
- Most patients recover with supportive management.
Prevention
- Multiparous females and donors with a history of transfusions are at highest risk for development of HLA antibodies, and in some countries their plasma is not used for clinical plasma, but only sent for fractionation.
- Donors who are implicated in TRALI should be deferred if leucocyte antibodies are identified.
Transfusion-Associated Circulatory Overload (TACO)
Pathophysiology TACO
- TACO is circulatory overload related to heart failure and results in pulmonary oedema.
- Onset of TACO may be related to underlying patient risk factors (e.g., cardiac or renal dysfunction) and/or transfusion-related risk factors (e.g., large volume, rapid rate of infusion).
- Some common risk factors for TACO include underlying congestive heart failure, chronic pulmonary disease, older age, receipt of continuous veno-venous haemodialysis, acute kidney injury, and patients who are receiving a large number of blood components.
Signs and symptoms
Investigation
- CXR (radiographic signs include new or worsening pleural effusions).
- If available, BNP and NT-pro-BNP can be helpful for diagnosis as increases in these values reflect ventricular distention, typically when the post-transfusion value is >1.5 times higher than the pre-transfusion value.
- Patients with TACO often respond to diuretic therapy; this may be a helpful distinguishing feature from TRALI which fails to respond.
The differential diagnosis includes:
- TRALI;
- Severe allergic or anaphylactic reaction.
Treatment
- Stop the transfusion.
- Position the patient upright (sit up position) for easier breathing.
- Maintain airway and give supplemental oxygen as needed.
- Diuretics.
- In cases of persistent severe hypoxaemia, intubation and mechanical ventilation may be necessary.
Prevention
- Attention to the patient’s fluid balance prior to the transfusion.
- Transfuse slowly within the allowable infusion rate.
- Transfuse one unit at a time and assess the patient after each unit.
- Consider administering diuretics to patients at high risk of fluid overload at time of transfusion.
Transfusion-Associated Dyspnoea (TAD)
Transfusion-associated dyspnoea (TAD) is defined as any respiratory distress within 24 h of transfusion that does not meet the criteria of TRALI, TACO or an allergic reaction and that cannot be explained by an underlying condition or any other known cause.
TAD remains a clinical diagnosis and a diagnosis of exclusion.
Pathophysiology
Sign and symptoms
- Dyspnoea < 24 h after starting transfusion.
- Hypoxaemia.
- Less frequent: chills, rigors or cough.
- No other associated signs of TRALI, TACO or other underlying causes or condition.
Treatment
Prevention
No specific measures provided other transfusion-related causes of dyspnoea have been ruled out.
DELAYED AND LATE ADVERSE EFFECTS OF TRANSFUSION
Key point 7: Recognition of a delayed adverse effect has implications for future transfusions. Taking a thorough transfusion history and careful laboratory documentation can help to prevent DHTRs.
Delayed haemolytic transfusion reaction
- DHTRs are haemolytic transfusion reactions that manifest 24 h or more following the transfusion.
- Given that they typically manifest days to weeks following transfusion, these reactions may be asymptomatic and/or go unrecognized.
Pathophysiology
- DHTRs are characterized by extravascular haemolysis in the spleen or liver (i.e., in contrast to intravascular haemolysis in AHTR). In most cases, the patient has been previously immunized due to pregnancy or transfusion. However, at the time of the index transfusion the antibody level has fallen below a detectable level, thereby resulting in a negative antibody screen or crossmatch. Following transfusion of a unit that is antigen-positive for the related antibody, a secondary immune response occurs and the antibody titre rises quickly in the patient, resulting in haemolysis over the following days.
- Anti-Kidd antibodies are particularly well-known for causing DHTRs.
Signs and symptoms
- Fever;
- Return of the patient’s haemoglobin to pre-transfusion levels without associated bleeding;
- Jaundice.
Investigations
- Recheck the patient’s blood group and antibody screen.
- Identify the underlying antibody if possible.
- DAT is usually positive and an eluate prepared from the red cells may show the new antibody
Treatment
Prevention
- Careful laboratory screening for red cell antibodies in the patient’s plasma and the selection of antigen-negative red cells.
- Keep an accurate and permanent blood bank record of the patients with antibodies and ensure there is a review process as part of pre-transfusion workup with every new request for a transfusion.
- Documentation of previous transfusion exposures by careful patient history. In some countries, antibody registers are used to identify patients with known antibodies, to improve the opportunity to provide antibody information and provide compatible blood.
- Educate the patient and provide written information so that they are aware of the reaction and the antibody and can provide valuable history in case they need further transfusions.
Transfusion-associated graft-versus-host disease (TAGvHD)
Pathophysiology
- Transfusion-associated graft-versus-host disease (TAGvHD) is a severe, usually fatal, immunological complication of transfusion.
- It most commonly occurs in immunocompromised patients. It is caused by immunocompetent donor T-lymphocytes that target the transfusion recipient cells. Unlike graft-versus-host disease in the bone marrow or solid organ transplant setting, all recipient tissues maybe targeted in TAGvHD, including the bone marrow, which eventually becomes aplastic. This accounts for the high fatality (nearly 100%) with TAGvHD.
- TAGvHD may also develop in situations where the donor is homozygous for a haplotype for which the recipient is heterozygous. Consequently, the transfused donor leucocytes are not rejected by the recipient as they are not recognized as being foreign. However, the donor lymphocytes recognize the recipient as foreign, leading to TAGvHD. TAGvHD typically occurs with a donation from a blood relative who shares an HLA haplotype; however, TAGvHD may also occur by chance, particularly in populations that are genetically homogenous.
Signs and symptoms
Onset of TAGvHD is usually 10–12 days following transfusion and includes:
- Fever;
- Skin rash and desquamation;
- Diarrhoea;
- Hepatitis;
- Pancytopenia.
Treatment
Prevention
- Gamma or X-ray irradiation of cellular blood products is the only very effective measure in routine use internationally at the time of writing to stop the proliferation of transfused donor lymphocytes. Pathogen inactivation technologies have also been shown to reduce the presence of viable donor lymphocytes, but clinical experience with these products to date is less than with irradiation as a preventive strategy.
- Leucoreduction of blood products is NOT effective in preventing TAGvHD and cannot be relied upon for risk reduction.
Iron overload
Iron overload is a frequent adverse effect associated with chronic transfusion support of patients with transfusion-dependent chronic anaemia. For example, patients with thalassaemia major, sickle cell disease or myelodysplasia treated with long-term blood transfusion are at high risk of developing iron overload.
Pathophysiology
Signs and symptoms
- Organ damage, especially of the liver, heart and endocrine system.
- The diagnosis is made based on the elevated ferritin levels and transfusion history, and presence of characteristic changes on imaging of affected organs.
Treatment and prevention
- Minimize the number of transfusions if possible. Consider alternatives where these exist, for example, disease-modifying therapies in myelodysplasia, or red cell exchange transfusion in sickle cell disease.
- Monitor for iron overload:
- Serum ferritin is the most widely available measure, but it is affected by many factors;
- Imaging with MRI is the most sensitive for assessment of cardiac and liver iron status.
- Chelation therapy with iron-binding agents, such as deferasirox, deferiprone or desferrioxamine, is widely used to prevent and treat iron overload in transfusion-dependent patients [6]. Timing of commencement of chelation depends on the clinical scenario. Specialist advice is recommended.
Post-transfusion purpura (PTP)
Post-transfusion purpura (PTP) is a rare adverse effect of transfusion.
Pathophysiology
- PTP refers to severe thrombocytopenia that is caused by a secondary immune response against platelet antigens. The implicated antibody varies by population. Any sensitizing event (e.g., transfusion or pregnancy) can lead to the development of these antibodies.
- Anti-human platelet antigen (HPA)-1a is typically implicated in White populations, while anti-HPA-3 and -4 are more commonly implicated in Asian populations. A typical history of PTP is that of an HPA-negative woman who has been immunized during a previous pregnancy and develops thrombocytopenia following transfusion due to presence of the cognate antigen in the donor blood.
- In PTP both the transfused antigen-positive platelets as well as the patient’s own antigen-negative platelets are also destroyed (i.e., bystander effect)
Signs and symptoms
- Acute, severe thrombocytopenia (of typically less than 10 × 109/L) approximately 2–14 days after transfusion.
- Signs of bruising and bleeding 2–4 weeks following transfusion if the platelet count falls below 50 × 109/L, with a danger of spontaneous bleeding at <10 × 109/L.
Treatment
- Intravenous Ig is the first choice of treatment.
- Avoid further transfusion of platelets unless risk of serious bleeding.
- If transfusion is necessary, give HPA-compatible platelets if available.
Prevention
TRANSFUSION-TRANSMITTED INFECTIONS
Key point 9: Prevention of TTIDs requires attention to all aspects of transfusion from donor selection, through collection practices, sterile/aseptic blood processing, laboratory testing and transfusion practices.
Many pathogens are transmissible by blood transfusion and should be addressed through donor risk-based deferral and/or laboratory-based screening (Tables 2, 3 and 4). Donors may not be aware of being infected. The following describes the major transfusion-transmitted infections (TTIs):
Worldwide
- HIV-1 and HIV-2
- Hepatitis B, C and E
- Syphilis (Treponema pallidum)
- HTLV-I
- Malaria (Plasmodium spp.)
- Chagas disease (Trypanosoma cruzi)
- Bacterial contamination (e.g., S. epidermidis, S. aureus): See ‘Bacterial contamination (septic transfusion reaction)’ section.
Table 2 Virus-related transfusion-transmitted infections.
|
Agent |
Pathology |
Modes of transmission* |
Epidemiology |
Recommended laboratory-based donor screening |
Other comments |
|
HIV-1 and 2 |
•Acute ‘seroconversion’ illness •Acquired immunodeficiency syndrome (AIDS) |
S, P, M |
Worldwide (HIV-1) West Africa (HIV-2) |
•Antibodies to HIV (anti-HIV 1/2) detectable approximately 18--21 days after infection. |
Combined Ag/Ab tests become positive approximately 15 days following infection |
|
HBV |
•Subclinical, many blood donors unaware of their status |
S, P, M, D |
•Worldwide, particularly in LMICs; highly prevalent in Asia and parts of Africa |
• HBsAg |
•Total anti-HBc may be considered in countries of low prevalence |
|
HCV |
•Most acute infection is asymptomatic •Chronic infection in 75%--85%; 10%--20% will develop cirrhosis ± hepatocellular carcinoma |
P, M, D |
•Major cause of chronic hepatitis worldwide. High prevalence: North Africa and Asia |
•Antibodies to HCV (long window period (up to 3 months) |
|
|
HEV |
•Mild and self-limiting in immunocompetent individuals |
F (ingestion of contaminated food (e.g. pork) or water); BT |
|
•Donor NAT has been implemented in a few high-income countries |
|
|
HTLV-I |
•Adult T-cell leukaemia (rare, delayed onset of many years) |
B, P, BT of cellular blood components |
High prevalence: southwest Japan, parts of Africa, the Caribbean and South America |
•Antibodies to HTLV-I in high prevalence areas |
Cell-associated virus, largely removed and transfusion prevented by leucoreduction |
*Bold type indicates major modes of transmission:
P: Parenteral (intravenous drug use, needle sticks, blood transfusion [BT]);
S: Sex with an infected partner;
M: Mother to child;
B: Breast feeding;
D: Direct contact with blood from an infected person;
F: Faeco-oral route.
Abbreviations: anti-HBc: hepatitis B core antibody; HbsAg: hepatitis B surface antigen; HBV: hepatitis B virus; HCV: hepatitis C virus; HEV: hepatitis E virus; HIV: human immunodeficiency virus; HTLV-I: human T-cell lymphotropic virus; NAT: nucleic acid testing; LMICs: low- and middle-income countries.
Table 3. Parasitic and bacterial transfusion-transmitted infections of global importance.
| Agent | Pathology | Modes of transmission* | Epidemiology | Recommended laboratory donor screening | Other comments |
| Treponema pallidum | Syphilis | S, P, M |
|
•Antibodies to T. pallidum antigen (treponemal specific test) •Nontreponemal serological test for syphilis |
National requirements vary. The FDA permits release of units from donors who have reactive nontreponemal screening test results and negative treponemal specific results |
| Plasmodium spp. | Malaria | Bite from mosquito (female anopheles); BT of cellular blood components |
•Widely endemic in tropical and subtropical areas. •3 billion people at risk of malaria •Data on TT malaria in LMICs are limited •TT malaria rare in non-endemic countries |
No standard of practice, proposed or applied approaches include: Endemic countries Non-endemic countries |
•In endemic countries, blood donors are frequently infected or have evidence of past malaria infection. This presents a complex challenge for transfusion services; therefore most blood for transfusion goes untested. •Malaria-type symptoms should be actively considered and investigated in transfusion recipients |
*Bold type indicates major modes of transmission:
P: Parenteral (intravenous drug use, needle sticks, blood transfusion);
S: Sex with an infected partner;
M: Mother to child;
B: Breast feeding;
D: Direct contact with blood from an infected person;
F: Faeco-oral route.
Abbreviations: BT, blood transfusion; FDA, US Food and Drug Administration; LMICs, low- and middle-income countries; TT, transfusion-transmitted.
Table 4. Regional pathogens or pathogens where blood transfusion has rarely or historically been implicated in transmission.
| Agent | Pathology | Transfusion Risk | Intervention |
| Babesia (B. microti) | Babesiosis | Regionally high; any red cell-containing blood product | Selected testing in high endemic areas (e.g., United States) with NAT or antibody tests |
| West Nile Virus, e.g., United States | Neuroinvasive disease | Regionally high | Selected testing in high endemic areas (e.g., United States) |
| Hepatitis A | Acute hepatitis | Low; case reports | Not indicated |
| CMV | Retinitis, enteritis, pneumonitis, colitis, disseminated infection |
Low; actual risk is an area of debate | Specific measures are not usually indicated.Options that have been used: •Leucoreduction - prestorage leucoreduction is highly effective, since CMV is primarily a cell-associated virus, although free CMV viraemia does occur and may be detected on donor testing, where performed; residual risk appears very low •Donor antibody testing with restriction of leukoreduced or seronegative cellular blood, products to high-risk recipients e.g., low-birth-weight infants (<1200 g), CMV-seronegative pregnant women, immunocompromised patients and CMV antibody-negative bone marrow recipients. National guidelines vary |
| Dengue virus | Haemorrhagic fever, dengue shock syndrome | Low; laboratory evidence of transmission but clinical cases of TT Dengue are very rare | Not indicated |
| Leishmania donovani and Leishmania infantum | Leishmaniasis | Low; case reports | Not indicated |
| Anaplasma phagocytophilum | Anaplasmosis | Low; case reports | Not indicated |
| Human parvovirus B19 | Aplastic crisis | Low; theoretical | Not indicated |
| Toxoplasma gondii | Toxoplasmosis | Low; historical case reports from granulocyte transfusions | Not indicated |
| vCJD | Spongiform encephalopathy | Low; theoretical | Not indicated for individual patient management; variable deferral for donors from high-risk countries introduced and ongoing in some settings |
Abbreviations: CMV, cytomegalovirus; NAT, nucleic acid testing; TT, transfusion-transmitted; vCJD, variant Creutzfeldt-Jakob disease.
General approach to TTIs
- Blood should be collected only from eligible donors as defined by national policy and standards for blood donation.
- Eligibility criteria for blood donation should be developed to minimize the risk of TTIs.
- The decision to implement laboratory-based donor screening should consider the risk of clinical infection and the regional prevalence of the infectious agent in the blood donor population.
- While the infection occurs at the time of the transfusion, serious manifestations of some TTIs may not occur until months, years or even decades later, such as cirrhosis and liver cancer from transfusion-transmitted hepatitis.
- Consider transfusion transmission as a potential route of infection when evaluating patients diagnosed with these infections, bearing in mind that some patients may not know they have been transfused.
At a minimum, all units of blood should be tested for:
- HIV-1 and HIV-2 (anti-HIV-1, anti-HIV-2) antibody;
- Hepatitis B (HBsAg) surface antigen;
- Hepatitis C antibody;
- Treponemal or non-treponemal antibody for syphilis.
Testing
- Tests for donor and donation screening should be validated and quality controlled.
- No blood product should be released for transfusion until all tests are shown to be negative without obtaining appropriate patient consent and notification of the treating physician.
- Any positive donation screening test should result in donor notification, counselling and referral for follow-up management.
- Blood from infected donors must be discarded.
Pre-seroconversion ‘window period’
- Refers to the period when the pathogen is present in the blood but antibodies to the infectious agent are not yet detectable.
- Antibody tests, when used alone, will not identify donors in the window period.
- Some viruses, notably HIV and HCV, have long serological window periods: nucleic acid testing or combination antigen/antibody (Ag/Ab) testing shortens the window period.
Modes of Transmission
P – Parenteral (intravenous drug use, needle sticks, blood transfusion)
S – Sexual contact with an infected partner
M – Mother to child
B – Breast Feeding
D – Direct contact with blood from an infected person
F – Faecal–oral route
Signs and symptoms
Bacterial contamination can result in acute sepsis in the recipient; see ‘Bacterial contamination (Septic transfusion reaction)' section. Viral infections can result in symptoms that can occur after many years and are related to the symptoms of the involved pathogen, for example, cirrhosis in case of hepatitis B or C.
Treatment and prevention
For bacterial contamination see ‘Bacterial contamination (septic transfusion reaction)’ section.
Prevention of TTIs can be accomplished through intervention at several phases of the process [7]:
- Donor selection based on interview and examination;
- Laboratory screening of donors;
- Blood product modification, for example, leucoreduction, or pathogen inactivation;
- Additional tests on products, for example, bacterial culture.
The treatment is specific to the infecting pathogen. In case of hepatitis anti-viral drugs can be used.
REFERENCES
- Proposed definitions surveillance non infectious adverse reactions 2011 (correction 2013). Available from: Haemovigilance Resources | The International Society of Blood Transfusion (ISBT) (isbtweb.org).
- Shot Reports. Available from: www.shotuk.org, see annual report.
- TRIP reports. Available from: https://www.tripnet.nl/en/publications/trip-reports/
- National healthcare Safety Network (NHSN) Biovigilance Component Hemovigilance Module Surveillance Protocol v2.8 Available from: https://www.google.nl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjxiY6RvZDhAhWF16QKHRuADpsQFjAAegQIABAC&url=https%3A%2F%2Fwww.cdc.gov%2Fnhsn%2Fpdfs%2Fbiovigilance%2Fbv-hv-protocol-current.pdf&usg=AOvVaw3w5lgsKHpE8qu46TyG0H7A.
- Lima A. Bloody Easy-Blood Administration. http://nurses.transfusionontario.org/; 2015.
- Shah FT, Porter JB, Sadasivam N, Kaya B, Moon JC, Velangi M, et al. Guidelines for the monitoring and management of iron overload in patients with haemoglobinopathies and rare anaemias. Br J Haematol. 2022; 196: 336–50.
- Busch MP, Bloch EM, Kleinman SH. Prevention of transfusion transmitted infections. Blood 2019; 133:1854–64.
THE AUTHORS
OTHER RESOURCES