INTRODUCTION
Circulating thrombocytes or platelets are anuclear discoid cells that are produced in the bone marrow from megakaryocytes during fragmentation. Thrombopoietin is the main growth factor controlling megakaryocyte production. The circulating life span of native platelets is approximately 10 days, and that of transfused platelets is approximately 3 days in a stable recipient. Platelets have receptors on their surface and granules inside, allowing them to participate in adhesion, aggregation and clot formation on the surface of injured endothelium, forming a haemostatic plug. The number of circulating platelets for an adult normally ranges between 150 × 109/L and 450 × 109/L. Decreased number of platelets (thrombocytopenia) refers to any situation where the patient’s platelet concentration is below 150 × 109/L, in an adult. Both thrombocytopenia and platelet dysfunction without thrombocytopenia can cause bleeding. Actively bleeding patients might require support from platelet transfusions. Platelets are also used for prophylactic transfusions to prevent bleeding due to decreased platelet production in patients undergoing chemotherapy, and/or haematopoietic stem cell transplantation (HSCT). In addition, platelets are transfused to some patients who have an increase in platelet destruction/consumption.
CAUSES OF THROMBOCYTOPENIA
Patients undergoing chemotherapy, HSCT, and those with chronic infections can have decreased platelet production. Another common cause of thrombocytopenia is an increase in platelet destruction/consumption that can have an immune or non-immune aetiology. Immune-mediated thrombocytopenia (ITP) is caused by different types of antibodies (autoimmune or alloimmune) that are produced to different platelet antigens and lead to elimination of the platelets in the spleen. Non-immune mechanisms cause platelet consumption and/or sequestration due to bleeding, enlarged spleen (sequestration) or vascular thrombi formation.
PROPHYLACTIC PLATELET TRANSFUSIONS
Prophylactic platelet transfusions in patients with hypoproliferative thrombocytopenia
Prophylactic platelet transfusions are routinely used in patients treated with chemotherapy and/or HSCT. They reduce the rate of spontaneous haemorrhagic events and improve clinical outcomes [1]. Although not yet a standard of practice, there is emerging evidence that prophylactic platelet transfusions are not necessary in autologous bone marrow transplant patients with hypoproliferative thrombocytopenia. The data suggest that this group of patients can be safely managed with therapeutic platelet transfusions only when bleeding occurs. The recommendation for prophylactic platelet transfusions in stable patients with hypoproliferative thrombocytopenia is to transfuse when the platelet count is 10 × 109/L or below. Multiple studies showed that, in stable patients with hypoproliferative thrombocytopenia, the platelet threshold above 10 × 109/L is adequate to minimize World Health Organization grade 2 or higher bleeding [2–4].
Prophylactic platelet transfusions in patients with dengue infection
Dengue infection is a major public health issue around the world, mainly in tropical and subtropical locations. Symptoms can vary from mild flu-like symptoms to more severe complications such as massive bleeding, shock and death. Thrombocytopenia is a common abnormal laboratory finding encountered in dengue infection, and bleeding is one of the complications that can occur. Different approaches, prophylactic versus therapeutic, have been studied in thrombocytopenic patients with dengue infection and the results are somewhat controversial. In addition, the optimal platelet threshold for prophylactic platelet transfusions is still being debated and evidence is still being generated in dengue patients [5]. However, at present, it is accepted that prophylactic transfusions should be given when the patient’s platelet count is 20 × 109/L or less [6,7], as platelet counts can decline very rapidly in this group of patients.
Prophylactic platelet transfusions in the setting of invasive procedures
Prophylactic platelet transfusions might be indicated in thrombocytopenic patients pre-procedure at specific platelet count triggers. They may also be required in patients with certain causes of platelet dysfunction, for example, aspirin, P2Y12 inhibitors (clopidogrel, prasugrel). There are multiple guidelines from different societies including multiple specialties (interventional radiology, thoracic surgery, haemostasis and thrombosis, haematology, blood transfusion) throughout the world on thresholds for prophylactic platelet transfusions for this group of patients. The established guidelines have some differences and there is some heterogeneity in the quality of evidence on which they are based but, overall, they are in line with each other. The recommended targeted thresholds for prophylactic platelet transfusions are listed below for some of the most common surgeries and procedures [9–13]. Platelet transfusions should be administered pre-procedure if platelet count falls below these targets:
- General surgery (nonneuraxial) < 50 × 109/L;
- Neurosurgery and ophthalmic surgery <100 × 109/L;
- Central venous catheter insertion < 20 × 109/L;
- Lumbar puncture < 50 × 109/L;
- Bone marrow biopsy < 20 × 109/L;
- Percutaneous liver biopsy < 50 × 109/L;
- Thorax centesis < 50 × 109/L.
Dose of prophylactic platelets
An adequate dose for prophylactic platelet transfusions is 1 apheresis unit or a dose of 4–5 pooled whole blood-derived platelet units for an average-size adult (70 kg). These doses result in a typical platelet increment in adults of 20,000–40,0000/μL. Apheresis platelets offer higher CCIs post-transfusion [8] but, overall, the two types of platelet products offer equivalent platelet survival and haemostatic effect in bleeding patients. In other words, higher CCIs with apheresis platelets do not translate into superior haemostatic effectiveness compared to whole blood-derived platelets.
One standard dose of platelets, either apheresis or a pool of whole blood-derived platelets, contains 3–4 × 1011 platelets on average. Interestingly, low (1.1 × 1011), medium (2.2 × 1011) and high doses (4.4 × 1011) of platelets transfused were equally effective in preventing bleeding in patients with hypoproliferative thrombocytopenia [3]. Patients in the low-dose category received platelet transfusions more frequently due to lower post-transfusion platelet increments compared to medium- and high-dose groups. High doses of platelets had no advantage in preventing bleeding compared to low- and medium-dose groups. In the study, the doses of platelets were adjusted for patient’s body surface area.
PLATELET TRANSFUSIONS IN THE BLEEDING PATIENT
Platelet transfusions can also be administered therapeutically for actively bleeding patients, but the optimal threshold for many settings is not known as few trials have addressed this issue. The typical platelet threshold for platelet transfusion in an actively bleeding patient is a platelet count below 50 × 109/L. In patients with cerebral haemorrhage, platelet transfusions should be given when platelet count is below 100 × 109/L. Independent of the platelet count, platelets should be administered to a bleeding patient with a known platelet dysfunction (such as a patient on antiplatelet medication or the surgery requires cardiopulmonary bypass). In addition, platelets are given in trauma resuscitation along with plasma and red blood cells, frequently as part of a major haemorrhage or massive transfusion protocol.
IDENTIFICATION AND MANAGEMENT OF PLATELET REFRACTORINESS
In patients, when a smaller than expected increase in the platelet count increment occurs after transfusion of an adequate dose of platelets, platelet refractoriness should be considered. One unit of random donor platelets derived from a unit of whole blood can increase the platelet count in an average size adult by 5,000–10,000/μL. Therefore, a dose of one random donor platelet per 10 kg of body weight is generally recommended. Pooled platelets prepared by pooling of 4–6 units of whole blood-derived platelets can give a post-transfusion increment of 20,000 to 40,000/μL in an average-size adult. The same increment would be expected from a dose of apheresis platelets.
There are two major causes of platelet refractoriness related to the patient: non-immune (80%) and immune (20%) causes. It is not uncommon to have a combination of both immune and non-immune causes.
When platelet refractoriness is suspected, a detailed review of the patient’s clinical history, along with a review of a history of platelet transfusions (how many transfusions and what increments were achieved), is indicated. To separate immune and non-immune causes of platelet refractoriness, a calculation of two consecutive CCIs is recommended. CCI is calculated 10–60 min post-platelet transfusion using the following formula:
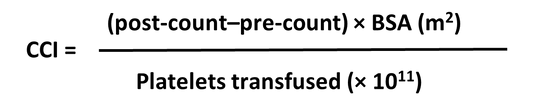
BSA = body surface area
The formula uses number of platelets transfused and body surface area to adjust CCI for these parameters. CCIs that are <7,500 after two consecutive transfusions define the immune refractory state.
Rules for calculating CCI and an example of calculation:
- Two consecutive CCIs;
- 10–60 min post-transfusion;
- A patient with body surface area of 1.7 m2 received a unit of apheresis platelets containing 4 × 1011 platelets, a post-transfusion count rose from 10,000 to 30,000/μL.
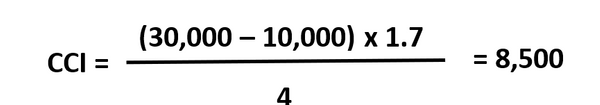
This is an example of CCI calculation. CCI is 8,500. The consecutive CCI is still 8,500. There is no evidence of immune refractoriness in this patient and non-immune causes should be considered.
While, overall, the major causes of platelet refractoriness are non-immune, the major immune cause of platelet refractoriness is HLA class I antibodies and they comprise approximately 90% of all immune causes. HLA antibody testing and HLA typing should be performed as part of the immune refractoriness evaluation. When HLA refractoriness is confirmed, HLA-matched platelets need to be obtained for future transfusion support, where these are available. HLA-matched platelets can only be collected by apheresis. HLA-A and HLA-B grade platelet matches provide the best platelet increments post-transfusion. Leucoreduction of blood components decreases the rate of HLA alloimmunization and the incidence of immune platelet refractoriness. Therefore, leucoreduced products should be transfused to patients requiring frequent transfusions, such as patients with haematological malignancy, HSCT and solid organ transplant recipients/candidates in order to prevent HLA alloimmunization.
Not all the HLA-matched platelet transfusions result in a successful post-transfusion platelet increment. In the majority of cases, the reason for that is overpowering non-immune mechanisms of platelet refractoriness. Therefore, immediate attention should be paid to these factors (splenomegaly, sepsis, fever, anti-fungal therapy, etc.) and appropriate therapeutic steps should be taken, if possible, to mitigate their impact on platelet refractoriness. Another reason for not responding to HLA-matched platelet transfusions may be the additional presence of HPA antibodies. Then, both HPA- and HLA-matched platelets may be necessary. As a temporary measure, while a search for HLA-matched platelets is still underway, providing transfusion support with whole blood-derived ABO-compatible platelets can be considered in case of bleeding.
Another technique that can be used to find compatible platelets in an HLA-alloimmunized recipient is the platelet crossmatch. Platelets from the platelet products are mixed with the patient’s plasma and compatible units are issued to the patient. This technique is fast and can be an effective alternative to HLA matching. However, it is not logistically feasible in highly immunized patients, since an extremely large number of platelet units would need to be crossmatched to find units that are compatible. Each of the techniques, HLA matching and crossmatching, has its advantages and disadvantages. The current evidence does not support the use of one technique over the other, and locally available resources are likely an important factor in determining which method will be utilized.
In an actively bleeding patient, when all the treatment options for non-immune causes of platelet refractoriness are exhausted and HLA-matched platelets are not available, other therapeutic options can be considered (antifibrinolytics, intravenous immunoglobulin) along with transfusions of non-HLA-matched platelets.
ABBREVIATIONS
CCI corrected count increment
DIC disseminated intravascular coagulation
HLA human leucocyte antigen
HPA human platelet antigen
HSCT haematopoietic stem cell transplantation
ITP immune thrombocytopenia
CONFLICT OF INTEREST STATEMENT
Alesia Kaplan: no disclaimers for this manuscript or conflict of interest.Darrell J. Triulzi: consultant to Fresenius Kabi.
Mark H. Yazer: no disclaimers for this manuscript or conflict of interest.
SUGGESTED READING
1.https://www.asco.org/practice-guidelines/quality-guidelines/guidelines/supportive-care-and-treatment-related-issues#/10171
2.http://www.aabb.org/programs/clinical/Pages/default.aspx
3.https://thorax.bmj.com/content/65/Suppl_2/i61
4.https://b-s-h.org.uk/guidelines/guidelines/use-of-platelet-transfusions/
5.https://www.brit-thoracic.org.uk/standards-of-care/guidelines/bts-guideline-for-diagnostic-flexible-bronchoscopy-in-adults/
REFERENCES
1. Stanworth SJ, Hudson CL, Estcourt LJ, Johnson RJ, Wood EM. Risk of bleeding and use of platelet transfusions in patients with hematologic malignancies: Recurrent event analysis. Haematologica. 2015;100:740–7.
2. Kumar A, Mhaskar R, Grossman BJ, Kaufman RM, Tobian AAR, Kleinman S, et al. Platelet transfusion: A systematic review of the clinical evidence. Transfusion. 2015;55:1116–27.
3. Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of Prophylactic Platelet Transfusions and Prevention of Hemorrhage. N Engl J Med. 2010; 362:600–13.
4. Stanworth SJ, Estcourt LJ, Llewelyn CA, Murphy MF, Wood EM. Impact of prophylactic platelet transfusions on bleeding events in patients with hematologic malignancies: A subgroup analysis of a randomized trial (CME). Transfusion. 2014;54:2385–93.
5. Lee TH, Wong JGX, Leo YS, Thein TL, Ng EL, Lee LK, et al. Potential Harm of Prophylactic Platelet Transfusion in Adult Dengue Patients. PLoS Negl Trop Dis. 2016; 10:e0004576.
6. Makroo RN, Raina V, Kumar P, Kanth RK. Role of platelet transfusion in the management of dengue patients in a tertiary care hospital. Asian J Transfus Sci. 2007; 1:4–7.
7. Thomas L, Kaidomar S, Kerob-Bauchet B, Moravie V, Brouste Y, King JP, et al. Prospective observational study of low thresholds for platelet transfusion in adult dengue patients. Transfusion. 2009; 49: 1400–11.
8.Triulzi DJ, Assmann SF, Strauss RG, Ness PM, Hess JR, Kaufman RM, et al. The impact of platelet transfusion characteristics on posttransfusion platelet increments and clinical bleeding in patients with hypoproliferative thrombocytopenia. Blood. 2012; 119:5553–62.
9. Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: A clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13.
10. Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl. 2):61–76.
11. Schiffer BCA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, et al. Platelet Transfusion for Patients With Cancer: Clinical Practice Guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2016;19:1519–38.
12. Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365–94.
13. Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax. 2013;68(Suppl. 1): i1–i44.
RESOURCES
THE AUTHORS


















