INTRODUCTION
Blood transfusion can be a life-saving measure for patients who experience haemorrhage, have severe anaemia or who are undergoing major surgery and cancer therapy. In high-income countries, the blood supply and associated resources are generally sufficient. However, in LMICs, the supply is far from secure to support the demand. Given the resource needs, time dependencies, and cold-chain requirements necessary to support a sustainable, safe and sufficient blood supply, attention to the health sector infrastructure is crucial (Box 1).
The blood supply relies on a system of interconnected parts that work in concert to assure safety and adequacy. From collections to testing to transfusion at the bedside, there are many parts that must work together to be effective. Blood systems may operate at a national level, where blood collection is coordinated under a centralized programme to manage the supply to many hospitals, or a single hospital may maintain a blood programme where it collects, tests and distributes blood to patients being cared for at the facility. However, even hospital-based blood programmes coordinate across multiple disciplines and apply a systems-thinking approach in order to ensure safety and availability. We reference blood systems, whether applied at a national or local level, to reinforce the continuous dynamic that is necessary to mitigate shortages, ensure accessibility, and improve safety no matter the scale. The WHO supports countries to develop nationally coordinated blood services, including implementing a national blood policy. Not all nations are yet able to achieve this, and in many cases blood supplies are still dependent on fragmented systems, with varying safety and quality standards [1].
The WHO describes five key objectives for blood services to ensure that blood is safe for transfusion [1]:
- The establishment of well-organized, nationally coordinated blood transfusion services to ensure the timely availability of safe blood and blood products for all patients requiring transfusion
- The collection of blood from voluntary non-remunerated blood donors from low-risk populations
- Quality-assured testing for transfusion-transmissible infections, blood grouping and compatibility testing
- The safe and appropriate use of blood and a reduction in unnecessary transfusions
- Quality systems covering the entire transfusion process, from donor recruitment to the follow-up of the recipients of transfusion.
DONOR RECRUITMENT
The challenges of blood transfusion systems in LMICs start with blood procurement and voluntary donor recruitment. Donor engagement is fundamentally based on raising public awareness through education campaigns as well as having equipment and motivated staff to work at blood drives and collection centres [2]. Often, secondary school students are targeted to be volunteer blood donors, which is advantageous because they are available and perhaps not yet sexually active, although when the school is not in session, the supply can be impacted. Another potential challenge for volunteer donation in some countries is that, in some regions, blood donation may be unfamiliar, depending on the cultural norms and the communities’ experience with healthcare. There are examples of using social media and church communities to engage and retain blood donors, although more research is needed in this area, including in LMICs [3, 4].
Because of the difficulty in maintaining adequate blood inventory of donations from voluntary non-remunerated repeat blood donors, many regions also use replacement donation which uses friends and family members as blood donors to replace the blood units used by a patient. Replacement donation models may not supply enough units, and - with their dependence on convenience donors - often will not yield a robust inventory that is available when needed to support emergency transfusions in patients with large blood needs or to support medical and elective surgical programmes. The WHO strongly supports a global framework for action to achieve 100% voluntary non-remunerated repeat blood donation and for countries to phase out family/replacement approach to donation [5]. Thus, in these settings, the ultimate aim should be to maximize conversion of low-risk voluntary and replacement donors into regular donors since those who are successful repeat donors have the best safety profile [6]. The ideal blood donor is a person who is healthy, and motivated to voluntarily donate repeatedly, irrespective of whether mandatory replacement is the official model of blood donation.
BLOOD COLLECTIONS
The collection of blood requires donor selection and deferral criteria to achieve the safest donors possible; this is best when standardized at the national level. The blood collection model will reflect the size and complexity of the recruitment model, which in turn depends on the projection of blood needs for the population served. Blood collections can occur at fixed sites (buildings) or via mobile collections such as specialized blood collection buses, or teams of collection staff that use space inside of other facilities. The process depends on many factors, including having appropriately trained staff resources [7]. Furthermore, in LMICs, ensuring the appropriate amount and type of collection equipment (needles, blood collection scales, beds or chairs), collection bags, sample tubes, labelling systems, storage at appropriate temperatures can be challenging, and can lead to having low collections when a key piece of equipment is not available or is broken [8]. Once whole blood is collected, manufacturing blood components, such as platelets, plasma and cryoprecipitate also depends on having the necessary equipment and supplies. Even when efficiencies of scale can be found, such as centralizing the procurement of blood bags, or location of blood product processing after collection, there must be a cold chain that can uphold temperature control requirements, monitoring and security of the supply. Production and supply chains also rely on the local infrastructure, weather conditions and geography.
INFECTIOUS DISEASE TESTING
The human immunodeficiency virus (HIV) epidemic in the late 20th century ushered in an era with enhanced focus on the prevention of transfusion-transmitted diseases. However, blood transfusion services in LMICs encounter many problems that impact the ability to perform testing, such as lack of funding, insufficient training, poor management, or failure in supply of reagents and consumables, and breakdown of the cold chain (mostly related to frequent electricity shortages). Supported by the WHO and other partners, there has been an international effort to provide testing for pathogens that are transmitted by blood transfusion and have a public health impact: HIV, hepatitis B (HBsAg), hepatitis C virus (HCV) and syphilis [9]. The prevalence of these diseases varies by region and by country, with high-income countries generally having lower rates than low-income countries (Table 1). Some centres in regions with a high prevalence of disease have attempted pre-donation screening in an attempt to save consumables, with mixed results that may depend on the setting and financial pressures specific to each region [10, 11]. The risk for bacterial contamination in platelets has also been documented in low-resource settings [12].
A potential future approach to improving the safety of the blood supply worldwide is pathogen reduction technology. By combining pathogen reduction technology with standard donor testing for infectious disease, the overall safety profile of the blood supply may be improved [13]. The decision to transfuse blood products is always a risk–benefit decision based on the patient’s clinical condition as well as the risk of infectious disease and other potential hazards of transfusion, the availability of blood, and costs of care.
Table 1. Proportions of blood donations with positive/reactive results on screening tests by World Bank income group; permission by WHO Blood Safety & Availability, 2016 [32].
| Income group by country | Proportion of blood donations with positive/reactive results, Median (interquartile range), % |
|||
| HIV | HBV | HCV | Syphilis | |
| High | 0.003 (<0.001–0.04) | 0.03 (0.008–0.18) | 0.02 (0.003–0.16) | 0.05 (0.005–0.26) |
| Upper middle | 0.08 (0.006–0.2) | 0.39 (0.16–0.69) | 0.21 (0.05–0.42) | 0.31 (0.12–1.07) |
| Lower middle | 0.20 (0.05–0.44) | 1.60 (0.94–4.13) | 0.40 (0.19–1.50) | 0.58 (0.18–1.47) |
| Low | 1.08 (0.56–2.69) | 3.70 (3.34–8.47) | 1.03 (0.67–1.80) | 0.90 (0.31–1.88) |
Abbreviations: HBV - hepatitis B virus; HCV - hepatitis C virus; HIV - human immunodeficiency virus
CLINICAL USE OF BLOOD
The care of patients needing and undergoing transfusion in LMICs can present significant challenges [14]. Blood shortages in sub-Saharan Africa can lead to preventable morbidity and mortality in children: in one study, 52% of severely anaemic children who did not receive blood transfusions died within 8 h of hospital admission, 90% died within 2.5 h [15]. The leading cause of maternal mortality is postpartum haemorrhage, accounting for 33.9% of maternal deaths, which the WHO estimates could be significantly decreased by improving the availability of blood [16, 17]. Blood shortages in LMICs are attributed to a combination of inadequate numbers of donations, blood wastage, inappropriate use of blood and blood components, and inadequate access to and use of pharmacological and nonpharmacological alternatives to allogeneic blood [18]. A substantial number of transfusions worldwide may be inappropriate: reports suggest that 23–52% of the transfusions in children and up to 90% of adult transfusions may be unnecessary [19, 20]. Needless transfusions compromise the availability of blood for patients who require it, and also expose patients to the risk of serious adverse transfusion reactions and transfusion-transmissible infections [21]. Inappropriate blood use can be caused by inadequate knowledge of the clinicians, leading to poor decision-making, as well as lack of access to alternative therapies.
The establishment of hospital transfusion committees can be pivotal in the support and supervision of transfusion services. Areas of focus for the committee may include developing and/or adapting evidence-based transfusion guidelines and pocket or electronic handbooks so they are readily available to clinicians, who should adhere to them in practice. For instance, there is now strong evidence that the early use of tranexamic acid (TXA) offers a survival benefit in bleeding patients who have been traumatically injured or are experiencing obstetric haemorrhage [22, 23]. TXA is inexpensive and safe. The use of TXA should be incorporated into local practice guidelines and should be readily available for rapid access and timely administration in critical bleeding scenarios.
Auditing and feedback of adherence to guidelines and bleeding management algorithms can be used as a tool to improve clinical care. An example of questionable common practice is the routine use of diuretics after transfusion; this may be inappropriate since the majority of patients who need transfusion present with hypovolaemia rather than heart failure as previously presumed [24]. Another example is that under-transfusion may be related to using a conservative transfusion policy, irrespective of haemoglobin threshold by guidelines. A recent randomized controlled trial found that immediate transfusion for African children with severe anaemia does not appear to impact long-term survival [25]. Further, there was no long-term survival effect when African children with severe anaemia <6 g/dL were randomized to a dose of blood (20 mL/kg vs. 30 mL/kg), although, in the presence of fever (>37.5°C) those transfused with the 30-mL/kg dose had lower survival compared to those transfused with a 20-mL/kg dose [26]. These studies show that additional research is needed to generate local evidence to drive practice on a number of these unresolved clinical questions. To evolve practice, translating evidence into policy and practice and effective engagement with clinicians about the clinical use of blood are important goals.
The bedside administration of blood, observation and clinical monitoring during the transfusion, and management of any adverse events due to transfusion are also important practices tied to transfusion safety [27, 28]. The availability of trained clinical staff - doctors, nurses, midwives, surgeons, anaesthesiologists, obstetricians, critical care and support staff - is important to provide appropriate management of a patient who is receiving a transfusion. Hospitals should have policies and procedures to direct the staff to carry out safe practices. Because modern transfusion devices are often lacking in under-resourced settings, it may be practical to consider alternatives. For instance, in settings where infusion pumps are not available, it is recommended to use a gravity infusion procedure to administer blood, and to ensure that the rate is approximately 5 mL/kg/h and completed within 4 h. However, there are instances where no alternatives may be appropriate, such as when blood infusion sets with a filter for small clots are not available. Under such difficult circumstances, clinicians should perform individualized risk–benefit assessment and transfuse in the safest manner possible.
Another option to minimize the reliance on donor blood is the use of cell salvage. The benefit of cell salvage, typically used in the operating room, is that it can be activated quickly, avoids exposure to donated blood and may address bleeding needs if availability of blood products is low or they are not available. The challenge of cell salvage is that trained staff are needed to use specialized equipment and consumables, which can be costly.
Patient blood management, a programmatic approach to minimize unnecessary transfusion, including through appropriate management of anaemia, offers a paradigm in which low-resource settings may improve the care of patients while also preserving the fragile available blood supply. Table 2 outlines recommended practices and those that should be avoided.
Table 2. Bedside transfusion practices
| Practice | Why is this important? |
| Strongly recommended practices | |
| Monitor the transfusion; before, during and after, including vital signs assessment | Best way to promptly identify transfusion reactions |
| Patient identification (right blood to the right patient) | Prevents incompatibility errors, which can be fatal |
| Post-transfusion haemoglobin assessment (4–8 h) | Promptly identifies under-transfused patients. The rates of under-transfusion are estimated to be 23–35% for most children with severe malarial anaemia; and may reach 50% among those with profound severe anaemia Appropriate re-transfusion is encouraged |
| Blood inventory management should not be hinged around blood storage age concerns | No evidence of harm, for in-date blood products, on grounds of storage age |
| Common practices with no evidence base | |
| Routine use of diuretics for every transfusion | No evidence of benefit |
| Routine warming of blood. (A common practice, often done without dedicated blood warming devices and for not the right indications) |
No evidence of benefit Rarely needed. When indicated (such as large volume rapid transfusions), use proper blood warmers to avoid haemolysing the blood |
| Routine use of anti-malarials with transfusions in malaria-endemic areas | No evidence of cost-effective benefit |
| Wrong and unsafe practices; strongly discouraged | |
| Thawing or warming blood in tap water | Risk of bacterial contamination |
Quality Systems
Ensuring the quality of blood and safe blood transfusion requires a systems approach that recognizes the integrated nature of the blood system [29]. Competent and capable staff are critical to this process. Screening of blood donors to protect their health and to prevent disease transmission to patients is of paramount importance. Laboratory testing protocols augment donor screening and should adhere to evidence-based methods or the manufacturer’s package insert. External quality assessment (EQA) or proficiency testing provides a means of independently verifying test system performance for blood typing and infectious diseases [30]. Some programmes offer discounts to participate in EQA for low-resource settings. If commercial EQA is not available, periodically testing blinded samples between laboratories is an alternative (Table 3).
Since blood comprises living cells and labile proteins, controlling the process of collecting, processing, transporting, and storing blood is critical to the quality of the components that will end up being transfused. Shipping containers should be appropriate for the environment where they are being used and validated to show the ability to maintain appropriate temperatures. If not continuously monitored, refrigerator and freezer temperatures should be regularly checked to maintain the quality of the blood. Documentation is essential to create a record to show that proper procedures were applied, or that corrective actions were taken when a process was found to be out of control. A practice of regularly reviewing such records and auditing activities provides reassurance that standard operating procedures are being followed. Finding and documenting shortcomings offers opportunities to engage in continual process improvement to enhance the safety and reliability of the blood system.
Examining ways to minimize blood product wastage in low-resource settings can help to conserve scarce resources (Table 4). In addition to expiry due to poor inventory management, in-process wastage can result if blood is not maintained at the appropriate temperature or if transportation over long distances adversely impacts time-dependent component preparation. Evaluating the supply chain for weaknesses and examining where timing constraints can be modified can be an effective tool in LMICs. Additionally, having back-up methods or alternatives assures redundancy in the event that the primary methods are not available.
Recognizing that blood is an integral part of the overall health delivery system, it is beneficial to forecast blood collection goals [31]. For example, if the health system grows oncology care capabilities, the demand for platelets could be anticipated. Connecting blood supply goals with health service projections ensures that the demand for blood, and consequently the supply of blood, is aligned (Figure 1).
Figure 1. Continuous linkage between blood supply and blood transfusion demand
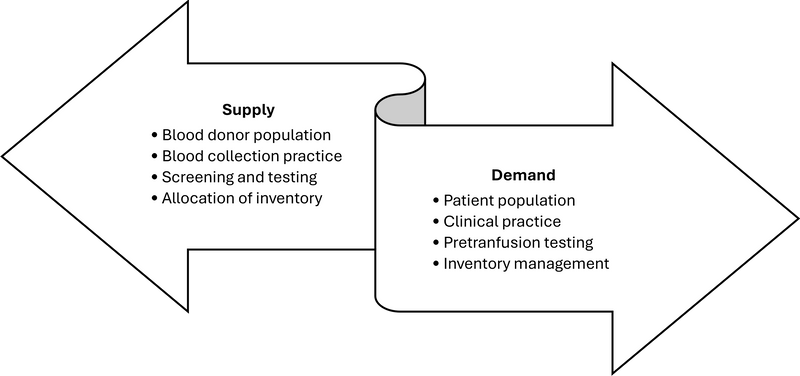
At the hospital level, governance, process control, education and monitoring are crucial to ensure blood safety. The hospital transfusion committee, which is lacking in many low-resource settings, is crucial to provide a framework for monitoring practice through setting evidence-based policies and taking steps to implement and monitor them to improve clinical practice and patient outcomes [32]. The committee should consist of stakeholders such as clinical care providers (doctors, nurses), laboratory staff, blood supplier staff and hospital administrators to review past performance and current issues. The committee should be chaired by a staff member with authority in the hospital setting to lead and effect necessary changes.
The transfusion committee can use published benchmarks and collaborations through professional transfusion societies as a source for best practice. Other critical activities of the hospital transfusion committee include setting policy and monitoring incident reporting systems for incidents caused by human error as well as adverse reactions caused by product. Hospital-based haemovigilance is a more advanced approach to incident monitoring in which the events can be tallied at the regional or national level for broader understanding of rare events that can, in turn, be used to set improved policies or testing algorithms (in the case of infectious disease transmission). The transfusion audit is a straightforward and low-cost approach to uncover quality issues in practice and documentation. Audit results should be reviewed by the transfusion committee on a regular basis, and action taken on relevant findings in order to improve practice.
Table 3. Quality systems considerations
| WHO objective | Method or approach | Considerations |
| Establish nationally coordinated blood transfusion service that can provide adequate and timely supplies of safe blood for all patients in need |
• National blood policies • Standard operating procedures |
How should resources be allocated and expended? How should activities be performed to meet the intended goal? |
| Collect blood from voluntary non-remunerated blood donors from low-risk populations and use stringent donor selection procedures |
• Donor screening targets the lowest risk-populations including repeat blood donors • Coordinated planning of blood collection and distribution with the healthcare system |
Who is suitable as a blood donor?
How to maintain an adequate supply? |
| Screen all blood for transfusion-transmissible infections and have standardized procedures in place for grouping and compatibility testing |
• Infectious disease testing • Pre-transfusion compatibility testing |
How to reduce the risk of transfusion-transmitted infection? How to determine if the blood product is the most compatible for the patient? |
| Assure appropriate clinical use of blood, including the use of intravenous replacement fluids and other simple alternatives to transfusion, where indicated |
• Multi-disciplinary transfusion committees • Transfusion guidelines and blood management principles |
How to be sure that the right blood goes to the right patient? How to maintain an adequate supply of blood and use it carefully? |
| Implement effective quality assurance measures for all aspects of the transfusion process, from donor recruitment and selection, through to infection screening, blood grouping and blood storage, to administration to patients and clinical monitoring for adverse events |
• Records • Audits, reviews • Laboratory participation in commercial EQA or blinded sample comparisons • Haemovigilance |
What happened and why? What can be improved? How does our testing system compare to other similar laboratories? How to examine risks of blood transfusion, including delays and under-transfusion? |
Abbreviations: EQA, external quality assessment; WHO, World Health Organization
Table 4. Measures to reduce blood wastage in low- and middle-income countries
| Consider plasma frozen within 24 h as an effective alternative to fresh frozen plasma. |
| Explore blood depots or decentralized distribution systems (hub and spoke) to minimize the risk of time constraints, temperature dependencies, and transportation challenges. |
| Maintain and exercise back-up alternatives for power outages and system down-time. |
| Align blood collection targets with health delivery system demands for blood transfusion. |
| Establish multi-disciplinary transfusion committees to review blood utilization and wastage, to inform feasible blood collection targets. |
THE FUTURE SUSTAINABILITY OF THE BLOOD SUPPLY
The sustainability of the blood supply in LMICs heavily relies on government commitment and ministries of health for funding appropriation and policies, such as whether blood is covered by national insurance, and the presence of a national regulatory authority for standard-setting and inspections [33]. Overall sustainability of the blood system should consider both the clinical needs and the resources available [34]. In low-resource scenarios, the margins are narrow and efficiency is important. Infrastructure critically impacts the blood supply, such as the state of the nation’s roads, facilities, communication and transportation.
Blood systems in developing countries are gradually moving from hospital-based blood banks, heavily dependent on replacement donors, or individuals who respond to an acute and emergent need for a given patient, to more consolidated and nationally supported systems that promote voluntary non-remunerated donation for the purposes of maintaining an ample inventory at a regional or national level. Repeat volunteer blood donors provide the safest source of blood because they have been educated, screened and tested, effectively reducing the risk of transfusion-transmitted disease and blood mistyping errors [32].
Securing adequate resources for sustainable blood services in LMICs that serve a distributed population is a major challenge. Over recent years, reductions in foreign aid have eroded the previously available resources used to support blood services. Thus, new or adapted financial resources or policies are needed for adequate blood services to exist in LMICs. The financial costs for blood services are difficult to estimate and the costs of preparing labile blood products seem to be on an increasing trend [34]. While the blood supply comes from the community, the demand for safe blood for transfusion occurs within the healthcare system. As countries strengthen their health services, fortifying the blood system to provide an adequate and safe supply of essential blood products is necessary. When health systems offer more services for cancer and non-communicable disease treatment, the demand for blood becomes a higher priority, relying on blood products such as platelets that have unique shelf lives, processing requirements, and infectious risks [35].
Blood transfusion practice in low-resource settings is executed best when considered as a systematic approach. Many LMICs have made great progress in achieving more regional or national coordination of blood services and in centralizing high-cost or complex activities, which can help provide safer, more standardized blood and blood products, more efficiently, although supply chain challenges remain an obstacle in some settings [36]. Education and policies can be used to guide use of evidence-based therapies and improve care within currently available resources.
SUGGESTED READINGS
Textbooks
- Murphy MF, Roberts DJ, Yazer MH. Practical transfusion medicine, 5th edition Hoboken, NJ: John Wiley & Sons Inc.; 2017
- Harmening D. Modern Blood Banking & Transfusion Practices, 7th edition Philadelphia: F.A. Davis; 2019.
- Hume HA. Blood Transfusion in Economically Restricted and Developing Countries. In: Shaz, editor. Transfusion Medicine and Hemostasis: Clinical and Laboratory Aspects, 3rd ed. Amsterdam: Elsevier; 2019: p. 349–355.
- Cohn CS, Delaney M, Johnson ST, Katz LM. AABB Technical Manual, 21st ed. Bethesda, MD: AABB Press; 2023
Journal articles
- Custer B, Zou S, Glynn SA, Makani J, Tayou Tagny C, El Ekiaby M, et al. Addressing gaps in international blood availability and transfusion safety in low- and middle-income countries: a NHLBI workshop. Transfusion. 2018; 58: 1307–17.
- Roberts DJ, Field S, Delaney M, Bates I. Problems and Approaches for Blood Transfusion in the Developing Countries. Hematol Oncol Clin North Am. 2016; 30: 477–95.
- Bates I, Chapotera GK, McKew S, van den Broek N. Maternal mortality in sub-Saharan Africa: the contribution of ineffective blood transfusion services. BJOG. 2008; 115:1331–9.
Resources
- World Health Organization. Blood Transfusion Safety: Universal access to safe blood and blood products for transfusion. 2010; Available from https://www.who.int/bloodsafety/en/
- World Health Organization. A Global Framework for Action: Towards 100% Voluntary Blood Donation. Geneva: International Federation of Red Cross and Red Crescent Societies; 2010: p. 123.
- World Health Organization. Safe Blood Components, for National Transfusion Authorities, D.o.E.H.T. Blood Transfusion Safety. Geneva: WHO; 2005.
- World Health Organization. Screening Donated Blood for Transfusion-Transmissible Infections: Recommendations for blood transfusion services Geneva; WHO; 2010.
- World Health Organization. Blood Safety and Availability. 2019. Available from https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability
- World Health Organization. Safe Blood and Blood Products: Distance Learning Materials & Modules. 2009.
REFERENCES
- World Health Organization. Blood Transfusion Safety: Universal access to safe blood and blood products for transfusion. 2010; Available from https://www.who.int/bloodsafety/en/.
- Bates I, Manyasi G, Medina Lara A. Reducing replacement donors in Sub-Saharan Africa: challenges and affordability. Transfus Med. 2007; 17:434–42.
- Piersma TW, Bekkers R, Klinkenberg EF, De Kort WLAM, Merz E-M. Individual, contextual and network characteristics of blood donors and non-donors: a systematic review of recent literature. Blood Transfus. 2017; 15: 382–97.
- Sumnig A, Feig M, Greinacher A, Thiele T. The role of social media for blood donor motivation and recruitment. Transfusion. 2018; 58: 2257–9.
- World Health Organization. A Global Framework for Action: Towards 100% Voluntary Blood Donation. Geneva: International Federation of Red Cross and Red Crescent Societies; 2010. p. 123.
- Asenso-Mensah K, Achina G, Appiah R, Owusu-Ofori S, Allain JP. Can family or replacement blood donors become regular volunteer donors? Transfusion. 2014. 54: 797–804.
- World Health Organization. Safe Blood Components for National Transfusion Authorities, D.o.E.H.T. In: Blood Transfusion Safety. Geneva: WHO; 2005.
- Dhingra N, Hafner V. [Safety of blood transfusion at the international level. The role of WHO]. Transfus Clin Biol. 2006. 13:200–2.
- World Health Organization. Screening Donated Blood for Transfusion-Transmissible Infections: Recommendations for blood transfusion services. Geneva: WHO; 2010.
- Clark KA, Kataaha P, Mwangi J, Nyamongo J. Predonation testing of potential blood donors in resource-restricted settings. Transfusion. 2005; 45: 130–2.
- Shittu AO, Olawumi HO, Adewuyi JO. Pre-donation screening of blood for transfusion transmissible infections: the gains and the pains - experience at a resource limited blood bank. Ghana Med J. 2014; 48:158–62.
- Hume HA, Ddungu H, Angom R, Baluku H, Kajumbula H, Kyeyune-Byabazaire D, et al. Platelet transfusion therapy in sub-Saharan Africa: bacterial contamination, recipient characteristics, and acute transfusion reactions. Transfusion. 2016; 56:1951–9.
- Nkohkwo A, Agbor G, Asongalem E, Tagny C, Asonganyi T. Whole blood pathogen reduction technology and blood safety in sub-Saharan Africa: A systematic review with regional discussion. Afr J Lab Med. 2016; 5: 363.
- Lund TC, Hume H, Allain JP, McCullough J, Dzik W. The blood supply in Sub-Saharan Africa: needs, challenges, and solutions. Transfus Apher Sci. 2013; 49:416–21.
- Kiguli S, Maitland K, George EC, Olupot-Olupot P, Opoka RO, Engoru C, et al. Anaemia and blood transfusion in African children presenting to hospital with severe febrile illness. BMC Med. 2015; 13: 21.
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006; 367: 1066–74.
- Bates I, Chapotera GK, McKew S, van den Broek N. Maternal mortality in sub-Saharan Africa: the contribution of ineffective blood transfusion services. BJOG. 2008; 115:1331–9.
- Osaro E, Charles AT. The challenges of meeting the blood transfusion requirements in Sub-Saharan Africa: the need for the development of alternatives to allogenic blood. J Blood Med. 2011; 2: 7–21.
- Nabwera HM, Fegan G, Shavadia J, Denje D, Mandaliya K, Bates I, et al. Pediatric blood transfusion practices at a regional referral hospital in Kenya. Transfusion. 2016; 56: 2732–8.
- Lackritz EM, Ruebush TK 2nd, Zucker JR, Adungosi JE, Were JB, Campbell CC et al. Blood transfusion practices and blood-banking services in a Kenyan hospital. AIDS. 1993; 7: 995–9.
- World Health Organization. Blood Safety and Availability. 2019. Available from https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability.
- CRASH-2 trial collaborators; Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010; 376: 23–32.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389: 2105–2116.
- Maitland K, Pamba A, Newton CR, Levin M. Response to volume resuscitation in children with severe malaria. Pediatr Crit Care Med. 2003; 4:426–31.
- Maitland K, Kiguli S, Olupot-Olupot P, Engoru C, Mallewa M, Saramago Goncalves P, et al. Immediate Transfusion in African Children with Uncomplicated Severe Anemia. N Engl J Med. 2019; 381: 407–19.
- Maitland K, Olupot-Olupot P, Kiguli S, Chagaluka G, Alaroker F, Opoka RO, et al. Transfusion Volume for Children with Severe Anemia in Africa. N Engl J Med. 2019; 381: 420–31.
- Mayaki Z, Kabo R, Moutschen M, Albert A, Dardenne N, Sondag D, et al. Knowledge, attitudes and clinical practice of blood products prescribers in Niamey. Transfus Clin Biol. 2016; 23: 78–85.
- Yudelowitz B, Scribante J, Perrie H, Oosthuizen E. Knowledge of appropriate blood product use in perioperative patients among clinicians at a tertiary hospital. Health SA Gesondheid. 2016; 15: 382–97.
- Roberts DJ, Field S, Delaney M, Bates I. Problems and Approaches for Blood Transfusion in the Developing Countries. Hematol Oncol Clin North Am. 2016; 30: 477–95.
- World Health Organization. Safe Blood and Blood Products: Distance Learning Materials & Modules; 2009. Available from https://www.who.int/bloodsafety/transfusion_services/Introductory_module.pdf?ua=1.
- van Hulst M, Smit Sibinga CT, Postma MJ. Health economics of blood transfusion safety–focus on sub-Saharan Africa. Biologicals. 2010; 38:53–8.
- Global status report on blood safety and availability 2016. Geneva: World Health Organization; 2017.
- Yetmgeta Abdella AM, Sajwani F, Pourfathollah AA, Smit Sibinga CT. Ensuring effective financing of national blood systems in support of universal health coverage. East Mediterr Health J. 2019; 25: 371–3.
- Tagny CT, Mbanya D, Tapko JB, Lefrère JJ. Blood safety in Sub-Saharan Africa: a multi-factorial problem. Transfusion. 2008; 48: 1256.
- Gallaher JR, Mulima G, Kopp D, Shores CG, Charles AG. Consequences of centralised blood bank policies in sub-Saharan Africa. Lancet Glob Health. 2017; 5: e131–e132.
- World Health Organization. Guidance on centralization of blood donation testing and processing; 2021. Available from https://apps.who.int/iris/handle/10665/340182
THE AUTHORS


















